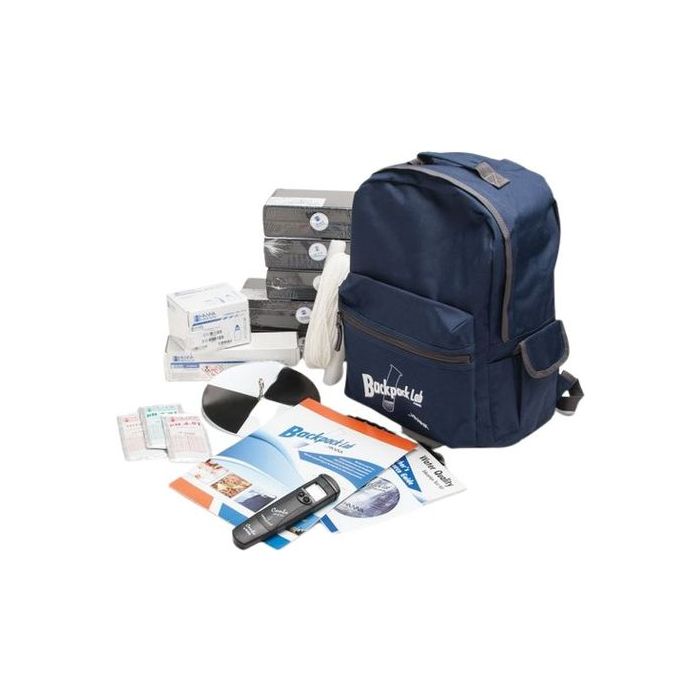
eXact® Micro 20 with Bluetooth® Well Driller Kit
R22,752.00 Excl. VAT
eXact® Micro 20 Photometer
eXact® Micro 20 with Bluetooth® Well Driller Kit
- Description
- Additional information
- Full test parameter list
- Video overview
Description
The eXact® Micro 20 with Bluetooth® is a powerful dual-wavelength photometer system that harnesses the technology to connect wirelessly to your smart device. The dual narrow band wavelength filters ensure delivery of the most accurate results! Pre-programmed to test over 40 water quality parameters with lab-quality accuracy Fast, accurate results with EZ-3 Method Test results can be shared in real-time via email or exported to a CSV spreadsheet.
Details:
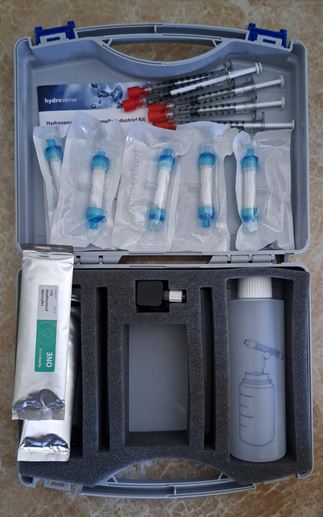
Large black plastic carrying case w/ Foam includes
Mini-dilution kit
eXact® Micro 20 Photometer
Cleaning brush
Instruction manual
25 tests of each
Total Alkalinity, pH, Free Chlorine (DPD-1), Combined/Total Chlorine (DPD-3), Total Hardness High, Phosphate, Copper, Manganese, Chloride (Salt), Total Iron, HR Chlorine, and Nitrate.
Additional tests are possible with a US$5 in-app purchase to activate each added parameter.
Drinking water FAQ
If I have a water measurement for total hardness and calcium hardness, can I calculate magnesium hardness or do I need other measurements such as TDS?
Yes, you can calculate magnesium hardness just from total hardness and calcium hardness measurements! Here’s how:
Magnesium Hardness = Total Hardness – Calcium Hardness
This works because in water chemistry, total hardness is primarily made up of just calcium and magnesium hardness. Both are typically expressed in the same units (usually mg/L or ppm as CaCO₃), making the subtraction straightforward.
You don’t need TDS or other measurements for this calculation, though those can be useful for other water quality assessments.
Would you like me to explain the underlying chemistry or help you with a specific calculation?
The health effects of chromium in well water depend on its form – chromium exists primarily in two oxidation states that have very different health impacts:
Chromium-6 (Hexavalent Chromium):
– Known carcinogen when inhaled
– Likely carcinogen when ingested
– Can cause liver and kidney damage
– Skin irritation/dermatitis from contact
– More mobile in groundwater
– More toxic form
– Often from industrial contamination
Chromium-3 (Trivalent Chromium):
– Essential nutrient in small amounts
– Much less toxic
– Poorly absorbed by the body
– Less mobile in groundwater
– Naturally occurring form
– Generally considered safe at typical levels
Key Considerations:
– EPA total chromium MCL: 0.1 mg/L (100 ppb)
– California specific Cr-6 standard: 0.01 mg/L (10 ppb)
– Most labs test for total chromium unless specifically requested
– Testing should specify which form is present
– Treatment options include:
– Ion exchange
– Reverse osmosis
– Reduction/coagulation filtration
Risk factors that increase concern:
– Proximity to industrial sites
– History of chrome plating operations nearby
– Acidic groundwater (can mobilize chromium)
– Geological formations high in chromium
The Sensafe (ITS) photometer when testing for “ammonia” is actually measuring total ammonia nitrogen (TAN), which includes both forms – the unionized ammonia (NH₃) and ionized ammonium (NH₄⁺) combined.
The test typically uses a Nessler method or salicylate method, where:
– Nessler method: Ammonia reacts with potassium iodide and mercury (II) chloride to form a yellow-brown complex
– Salicylate method: Ammonia reacts with salicylate and hypochlorite to form a blue-green complex
The photometer doesn’t distinguish between the two forms (NH₃ and NH₄⁺) – it gives you the total concentration, usually expressed as mg/L NH₃-N (ammonia as nitrogen) or just mg/L NH₃.
If you need to know the specific amount of unionized ammonia (NH₃) or ammonium (NH₄⁺), you would need to:
1. Take this total reading
2. Measure the water’s pH and temperature
3. Use these values in equilibrium calculations to determine the relative proportions
Yes, you can calculate ammonium (NH₄⁺) from ammonia (NH₃) levels, but you need to know the pH and temperature of the water since these factors determine the equilibrium between ammonia and ammonium.
The relationship follows this equilibrium:
NH₃ + H₂O ⇌ NH₄⁺ + OH⁻
The calculation uses the Henderson-Hasselbalch equation:
pH = pKa + log([NH₃]/[NH₄⁺])
Where:
– pKa varies with temperature (approximately 9.25 at 25°C)
– [NH₃] is the ammonia concentration
– [NH₄⁺] is the ammonium concentration
You can rearrange this to solve for ammonium:
[NH₄⁺] = [NH₃] × 10^(pKa – pH)
At typical drinking water pH (6.5-8.5), most of the total ammonia exists as ammonium (NH₄⁺). For example:
– At pH 7, about 99% exists as NH₄⁺
– At pH 8, about 95% exists as NH₄⁺
– At pH 9.25 (the pKa), it’s a 50/50 split
What would the permanganate test of water tell us?
The permanganate test (often called Permanganate Value or PV test) measures the amount of organic matter in water by using potassium permanganate (KMnO4) as an oxidizing agent. Here’s what it tells us:
Primary Information:
– Indicates the level of organic contamination in the water
– Measures oxidizable organic matter content
– Gives an indication of the oxygen demand of the water
The test works because:
– KMnO4 oxidizes organic compounds in the water
– The purple permanganate is reduced to colorless Mn²⁺
– The amount of permanganate consumed corresponds to the amount of oxidizable material present
Typical Applications:
1. Water treatment plant monitoring
2. Assessing raw water quality
3. Checking treatment efficiency
4. Indicating potential for disinfection byproduct formation
Limitations:
– Not all organic compounds are equally oxidized
– Doesn’t specifically identify the types of organic matter
– Some inorganic substances can also consume permanganate
– Results can be affected by temperature and pH
A high permanganate value typically suggests:
– Higher organic pollution
– Potentially poor water quality
– May need more intensive treatment
– Could indicate contamination from sewage or industrial waste
Aluminum in drinking water can pose health risks, but the situation is nuanced and depends on concentration levels. Here’s what’s important to know:
Health Considerations:
– The main concern is potential neurotoxicity, particularly for vulnerable populations
– Studies have suggested possible links to Alzheimer’s disease, though this relationship remains debated in the scientific community
– Young children, elderly people, and those with kidney problems may be more sensitive
Safety Guidelines:
– The WHO has not established a health-based guideline value due to limitations in available data
– The U.S. EPA recommends a secondary (non-enforceable) standard of 0.05-0.2 mg/L (50-200 μg/L)
– The practical limit is often set at 0.2 mg/L based on aesthetic concerns (water discoloration)
Sources in Drinking Water:
– Natural presence in soil and rocks
– Water treatment processes (aluminum-based coagulants)
– Industrial contamination
– Acid rain can increase aluminum mobilization from soil
It’s worth noting that:
– Most healthy adults can safely process the levels typically found in treated drinking water
– The amount of aluminum from drinking water is usually much lower than what we get from food and other sources
– Modern water treatment plants carefully control aluminum levels when using aluminum-based coagulants
If you’re concerned about aluminum levels in your drinking water, you can:
1. Request a water quality report from your local utility
2. Have your water tested, especially if you use a private well
3. Consider using a water filter certified for aluminum reduction if levels are high
Let me break down the health implications and typical standards for each of these elements in drinking water:
Bromine:
– Rarely found in pure form in drinking water
– Usually present as bromide ions or brominated compounds
– Maximum contaminant level (MCL) isn’t set for bromine itself
– Main concern is formation of disinfection byproducts (DBPs) when water is chlorinated
– Brominated DBPs are generally considered more toxic than their chlorinated counterparts
Cyanide:
– Highly toxic even in small amounts
– WHO guideline: 0.07 mg/L
– EPA MCL: 0.2 mg/L
– Acute exposure can be lethal
– Sources include industrial contamination (mining, electroplating, steel manufacturing)
– Natural cyanide-producing bacteria can be present in some water sources
– Regular monitoring is required for public water systems
Fluoride:
– Beneficial in small amounts for dental health
– WHO guideline: 1.5 mg/L
– EPA MCL: 4.0 mg/L
– EPA secondary standard (for cosmetic effects): 2.0 mg/L
– Too little: increased risk of dental caries
– Too much: can cause dental fluorosis (discoloration of teeth)
– Very high levels can cause skeletal fluorosis
– Many water systems add fluoride for dental health benefits
– Optimal fluoridation level for dental health: 0.7 mg/L (US Public Health Service recommendation)
What is the difference between total alkalinity, bicarbonate alkalinity, carbonate alkalinity, M alkalinity and P alkalinity?
Let me break down these different types of alkalinity in water chemistry:
Total Alkalinity (TA) is a measure of all alkaline substances in water that can neutralize acids. It’s the sum of all bases that are titratable with a strong acid to a pH of 4.5. This includes:
– Bicarbonates (HCO₃⁻)
– Carbonates (CO₃²⁻)
– Hydroxides (OH⁻)
– And sometimes minor contributors like borates, phosphates, and silicates
M Alkalinity (Methyl Orange Alkalinity) is essentially the same as Total Alkalinity, as it’s measured by titrating to the methyl orange endpoint (pH 4.5). This measures all alkaline substances.
P Alkalinity (Phenolphthalein Alkalinity) is measured by titrating to the phenolphthalein endpoint (pH 8.3). This measures:
– All hydroxide alkalinity
– Half of the carbonate alkalinity
– None of the bicarbonate alkalinity
Carbonate Alkalinity refers specifically to the CO₃²⁻ contribution to alkalinity. It can be calculated as:
Carbonate Alkalinity = 2(P Alkalinity – [OH⁻])
Bicarbonate Alkalinity refers to the HCO₃⁻ contribution. It can be calculated as:
Bicarbonate Alkalinity = Total Alkalinity – 2(Carbonate Alkalinity) – [OH⁻]
The relationships between these forms depend on pH:
– Below pH 4.5: No significant alkalinity
– pH 4.5-8.3: Primarily bicarbonate
– pH 8.3-9.6: Mix of bicarbonate and carbonate
– Above pH 9.6: Primarily carbonate and hydroxide
Additional information
| Weight | 3 kg |
|---|---|
| Dimensions | 40 × 30 × 15 cm |
eXact® Micro 20 Photometer test capability
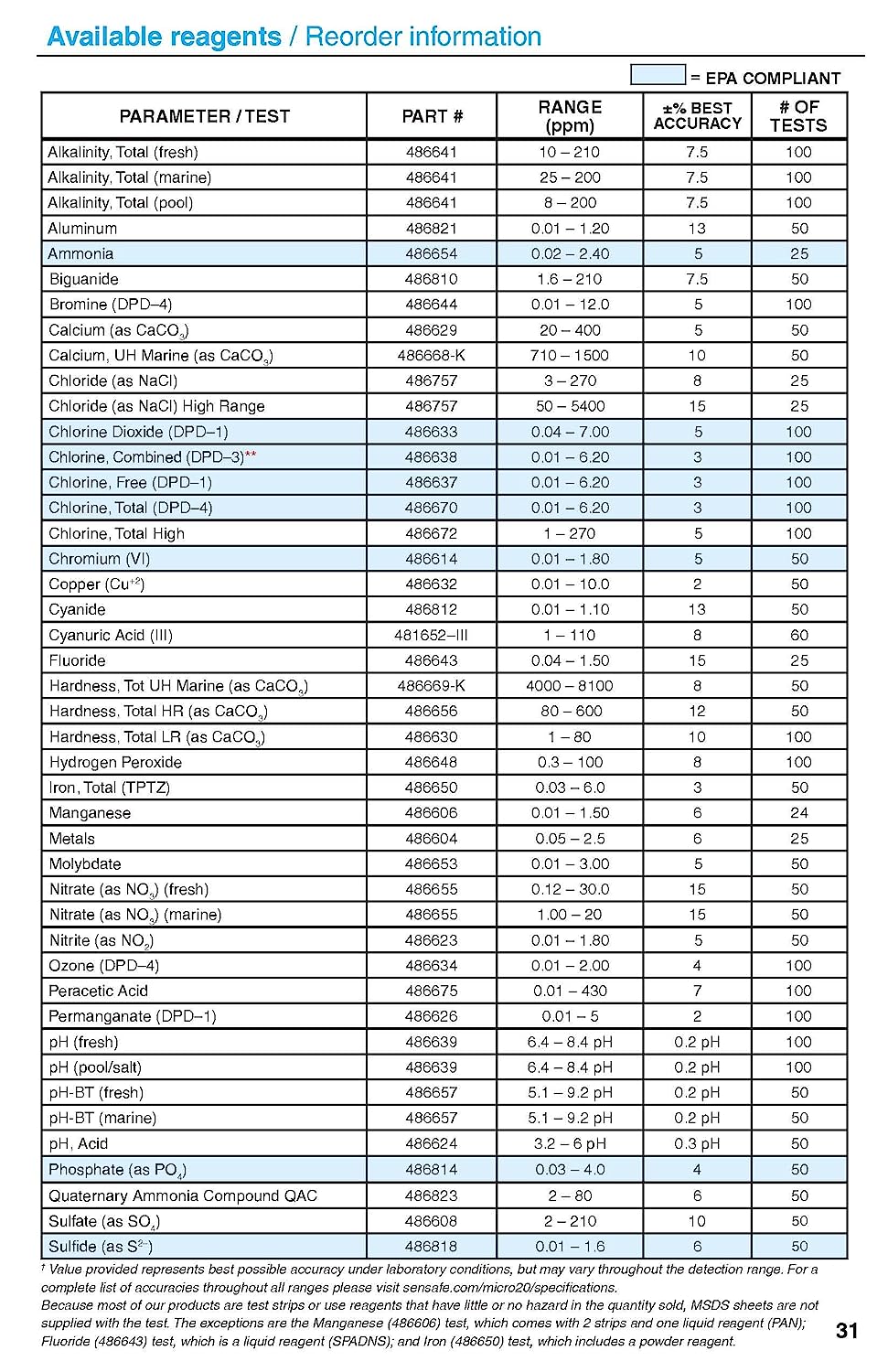
The testing capability requires a consumable for each parameter to be tested. The tests per bottle are listed above.
Parameter Part Number Range (ppm) *Best Accuracy ±%
Alkalinity, Total (fresh) 486641 10 – 200 10
Alkalinity, Total (marine) 486641 10 – 175 10
Alkalinity, Total (pool) 486641 20 – 200 10
Aluminum 486821 0.01 – 1.20 13
Ammonia 486654 0.02 – 2.40 12
Biguanide 486810 1.6 – 210 10
Bromine 486644 0.04 – 10.0 10
Calcium (as CaCO3) 486629 20 – 400 12
Calcium, Ultra High (marine) 486668-K 710 – 1400 10
Chloride (as NaCl) 486757 3 – 270 10
Chloride (as NaCl) HR 486757 50 – 5400 15
Chlorine Dioxide (DPD-1) 486633 0.04 – 6.50 9
Chlorine, Combined (DPD-3) 486638 0.01 – 6.20 6
Chlorine, Free (DPD-1) 486637 0.01 – 6.20 6
Chlorine, Total High 486672 1 – 200 10
Chlorine, Total (DPD-4) 486670 0.01 – 6.20 6
Chromium (VI) 486614 0.01 – 1.80 12
Copper (Cu+2) 486632 0.01 – 10.0 8
Cyanide 486812 0.01 – 1.10 13
Cyanuric Acid 481652-III 1 – 110 12
Fluoride 486643 0.04 – 1.30 15
Hardness, Total HR (as CaCO3) 486656 60 – 600 12
Hardness, Total LR (as CaCO3) 486630 1 – 80 10
Hardness, Tot UH Marine Kit (as CaCO3) 486669-K 5000 – 8000 8
Hydrogen Peroxide 486648 0.3 – 100 8
Iron, Total (TPTZ) 486650 0.03 – 6.0 12
Manganese 486606 0.02 – 1.40 8
Metals (+2 Valence) 486604 0.02-1.8 10
Molybdate (MoO4) 486653 0.01 – 3.00 10
Nitrate (as NO3) 486655 0.12 – 30.0 20
Nitrate (as NO3) (marine) 486655 1 – 20 15
Nitrite (as NO2) 486623 0.03 – 1.80 8
Ozone (DPD-4) 486634 0.01 – 2.00 8
Peracetic Acid 486675 1-380 7
Permanganate (DPD-1) 486626 0.02 – 5 10
pH (fresh) 486639 6.4 – 8.4 pH 0.2 ph
pH (pool) 486639 6.4 – 8.4 pH 0.2 ph
pH, Acid 486624 3.2 – 6.2 pH 0.3 ph
pH-BT (fresh) 486657 5.1 – 9.2 0.2 pH
pH-BT (marine) 486657 5 – 9.2 0.2 pH
Phosphate 486814 0.03 – 4 8
Quaternary Ammonia (QAC) 486823 2 – 80 8
Sulfate 486608 2 – 210 10
Sulfide 486818 0.01 – 1.6 6
LOOK UP TABLE REQUIRED
Turbidity 4 – 900 NTU 11
*Value provided represents best possible average accuracy under laboratory conditions, but may vary throughout the detection range.
Video overview – usage
Precision